-40%
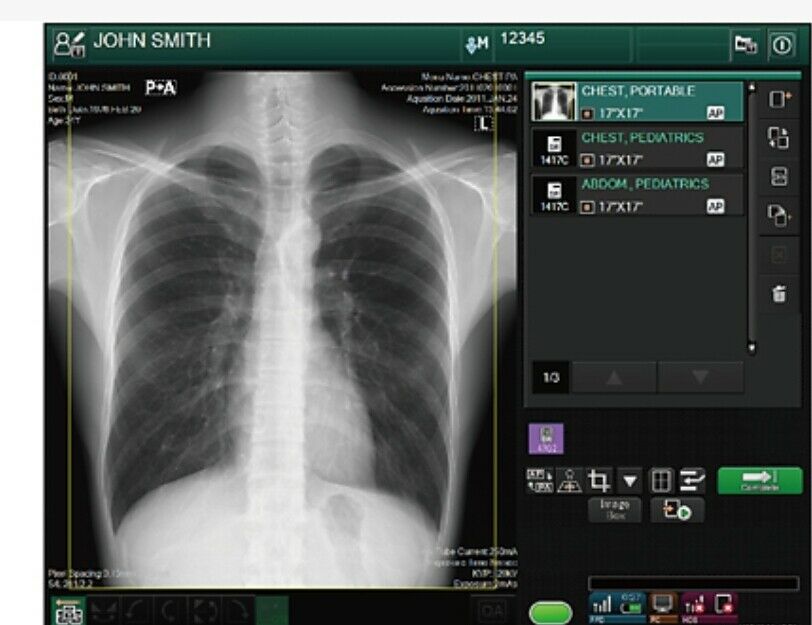
FDR Go2-flex, Fujifilm’s ‘Hybrid’ DR Portable xray machine
$ 14079.64
- Description
- Size Guide
Description
2012 FUJI GO2 Flex with wireless DR panel and cr cassettes.2021 software setup
"The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item."
Mobile-CR LLC, Chicago IL Ph: 2623445999
please ask questions : [email protected] Legal Information Sale includes only items and accessories explicity shown or listed in the text of this advertisement. Additional accessories, features, and items are not included. we warrant the item to be in the condition described, but makes no guarantee regarding compatibility with other equipment, software, or use other than the item's original intended use. This agreement will be governed by the laws and any disputes arising from this agreement shall be decided exclusively by the district or magistrate state courts in the Illinois. Any party that unsuccessfully challenges the enforceability of this Choice of Law and Forum Selection clause shall reimburse the prevailing party for its attorney fees and costs associated with defending against the challenge of said clause. All devices are thoroughly cleaned to manufacturer specifications and inspected before listed for sale. The sale of this item may be subject to strict regulation by the US Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an authorized purchaser. If the item is subject to state or FDA regulation, you must provide verification that you